INNOVATION
Prosthetics Care Tests a New Digital Blueprint
New VA pilots, HP backed initiatives, and Medicare recognition advance digital prosthetics
12 Dec 2025

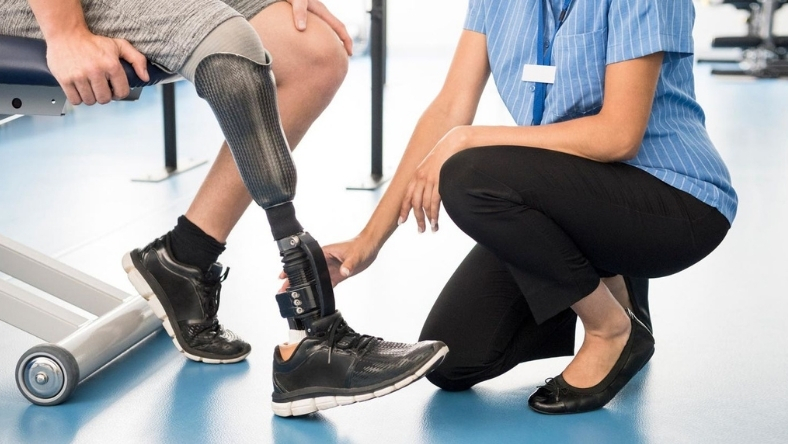
A gradual shift towards digital manufacturing is under way in the US prosthetics sector, as clinics and health systems begin to integrate 3D printing and data-driven design into what has long been a manual craft.
The latest signal came in 2025, when the US Department of Veterans Affairs said the VA Puget Sound Health Care System had successfully piloted an end-to-end digital workflow for producing a transtibial prosthetic socket. Clinicians scanned a patient’s residual limb, generated a digital model and manufactured the socket on site using additive manufacturing. While limited in scope, the project showed how digital processes could shorten production times and improve consistency.
The VA initiative reflects a broader pattern of experimentation rather than wholesale change. Most US prosthetic clinics still rely on traditional fabrication methods, but larger providers and academic centres are testing digital tools to assess their clinical, operational and financial impact. Early findings suggest that storing and refining digital files can help clinicians adjust fit over time without restarting production, potentially reducing waste and repeat visits.
Technology suppliers are supporting these trials through partnerships rather than direct market control. HP, whose polymer 3D printing systems are used across industries, has worked with healthcare organisations to test materials, validate workflows and explore reimbursement models. Coverage in Plastics Today has highlighted HP’s role in collaborative pilots aimed at meeting clinical standards, rather than replacing existing providers.
Policy changes have also helped to lower barriers. Medicare has now formally recognised 3D printing within its coding and reimbursement framework, a step widely viewed as necessary for broader clinical adoption. Clearer payment pathways make it easier for hospitals and clinics to justify investment in equipment, staff training and digital infrastructure.
Significant challenges remain. Smaller practices often lack the capital and technical capacity to adopt new systems, and industry standards for digital quality control are still evolving. Researchers and professional bodies say these issues can be addressed through shared guidance, training programmes and continued regulatory clarity.
As evidence accumulates and tools become more accessible, digital manufacturing is expected to play a larger role in prosthetic care. The pace of change may be incremental, but the direction is increasingly clear.
Latest News
27 Jan 2026
From Solo Bots to Team Players in Healthcare26 Jan 2026
Serve expands into hospitals with Diligent acquisition22 Jan 2026
Health Systems Point to a New Era in Prosthetics Care20 Jan 2026
In Prosthetics and Robotics, Bigger Is Becoming Better
Related News

PARTNERSHIPS
27 Jan 2026
From Solo Bots to Team Players in Healthcare

INVESTMENT
26 Jan 2026
Serve expands into hospitals with Diligent acquisition
MARKET TRENDS
22 Jan 2026
Health Systems Point to a New Era in Prosthetics Care
SUBSCRIBE FOR UPDATES
By submitting, you agree to receive email communications from the event organizers, including upcoming promotions and discounted tickets, news, and access to related events.